Research and Development of Hyper Dry human amniotic membrane (HD-AM)
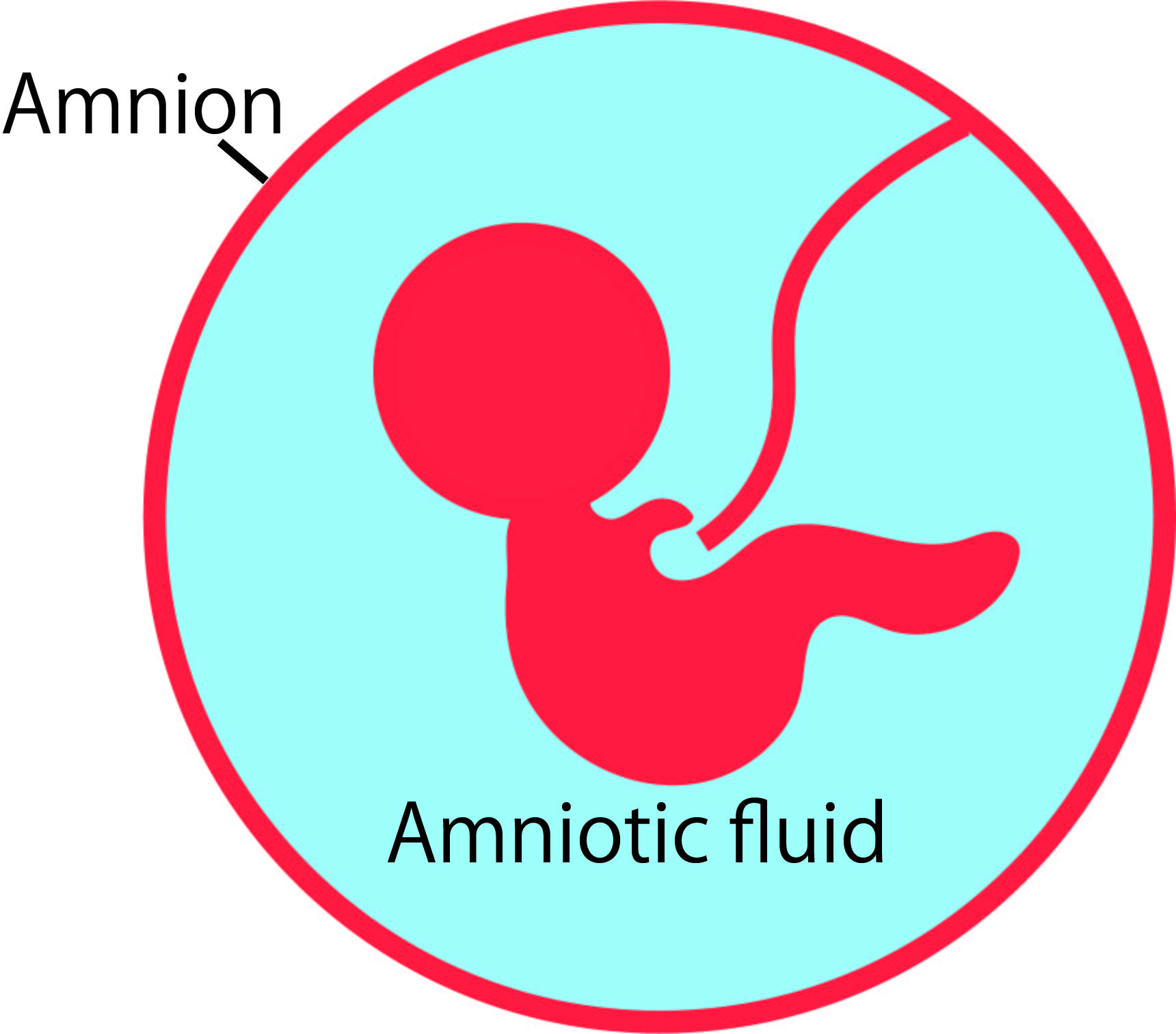
The amniotic membrane is a thin membrane that surrounds the fetus and the amniotic fluid. The baby grows floating in the amniotic fluid, and the amniotic membrane is a strong membrane made of collagen that holds the amniotic fluid in place (Figure 1A).
We are researching and developing this amniotic membrane as a regenerative medical material. Amniotic membrane has long been used to treat wounds and burns. In recent years, the WHO has also approved it for use in eye treatments only, and it has become used worldwide. In Japan, a treatment for intractable ocular surface diseases using cryopreserved amniotic membrane has been covered by health insurance since April 2014. With this method, amniotic tissue (cells) are transplanted to the affected area while still alive, so antibiotics are used to prevent infection, but they cannot prevent viruses.
To solve this problem, we have developed Hyper-Dry Human Dried Amniotic Membrane (HD-AM) by drying the amniotic membrane using a special method called Hyper-Dry and sterilizing it with gamma rays. The Hyper-Dry method is a drying method that controls three factors: 1) vacuum, 2) far-infrared rays, and 3) microwaves. It is used in product development in the food industry because it “retains the aroma”and “unlike freeze-drying, it can dry large foods such as bananas and apples.” By using the Hyper-Dry method, we have been able to create an excellent dried amniotic membrane that retains the excellent characteristics of a living amniotic membrane.
In order to apply amniotic membrane to patient treatment, we have been conducting basic research using scientific research funds from the Ministry of Education, Culture, Sports, Science and Technology to investigate how to increase its strength, shape it, and its effectiveness against microorganisms such as bacteria and viruses, as well as its physical properties (strength, material permeability, light permeability, changes in appearance, water content, and collagenase resistance) with the aim of pharmaceutical development.
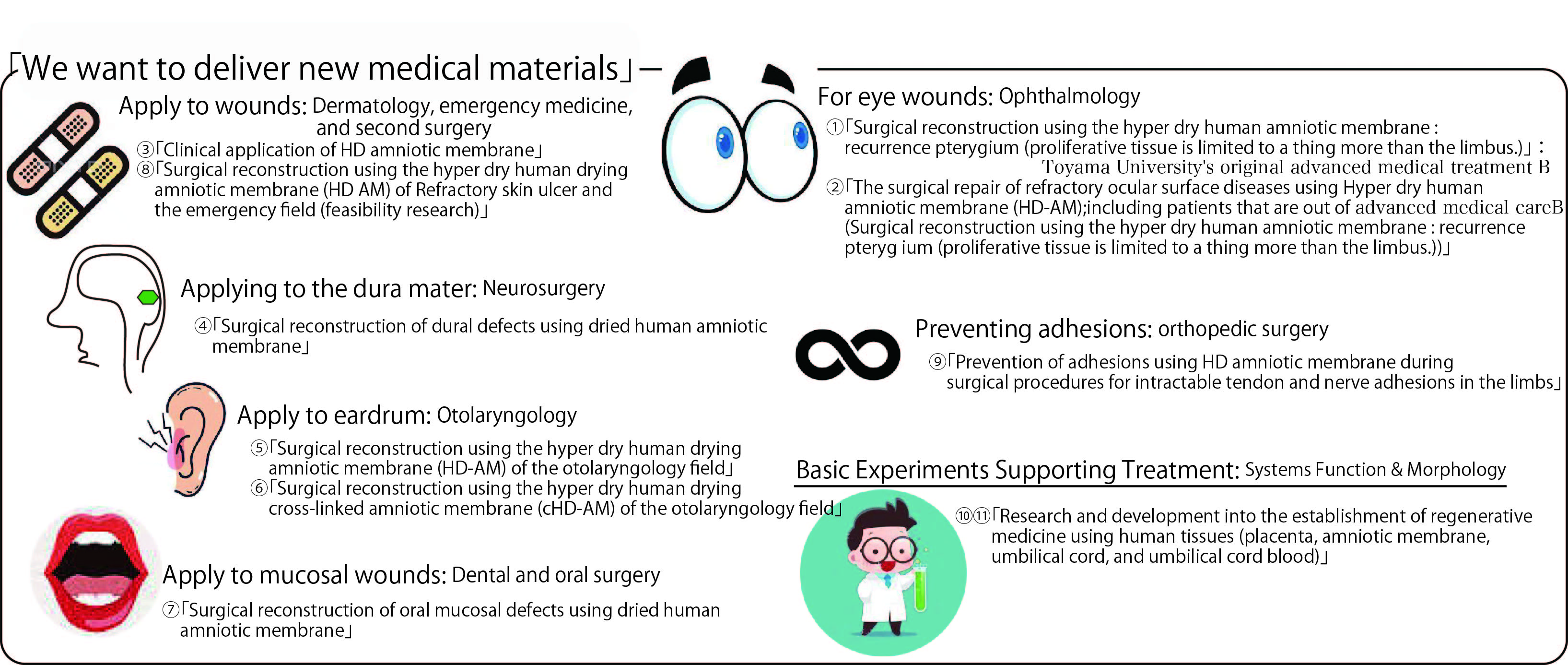
On the other hand, from 2005 to September 2020, when we were a department of regenerative medicine, Ophthalmology, Dermatology, Neurosurgery, Otolaryngology, Dentistry and Oral Surgery, and the Second Department of Surgery conducted joint clinical research and achieved good results (Figure 1B). In particular, the Ophthalmology Department is conducting Advanced Medical Treatment B using HD-AM at 11 university hospitals around the country, mainly at Toyama University Hospital. We are also conducting specific clinical research with the cooperation of the Otolaryngology and Emergency Medicine/Dermatology departments.
With support from Toyama University through the Drug Discovery and Healthcare Project (Engineering Healthcare), an industry-academia collaboration activity, we are also proceeding with safety testing as instructed by the Pharmaceuticals and Medical Devices Agency (PMDA), which also shows Toyama University's "seriousness" in obtaining approval as a medical device.
Together with Sakura Seiki Co., Ltd., we aim to commercialize this technology using our patents and know-how.
Thanks to the cooperation of so many people, we have been able to take over the theme from the former Regenerative Medicine Department and continue the development of regenerative medicine using amniotic cells and HD-AM.
Thank you very much.
NEWS
- 2025.05.30
- I've edited the text
- 2023.04.01
- The site has been published